PPB warns of fake cancer and kidney transplant drugs after WHO alert
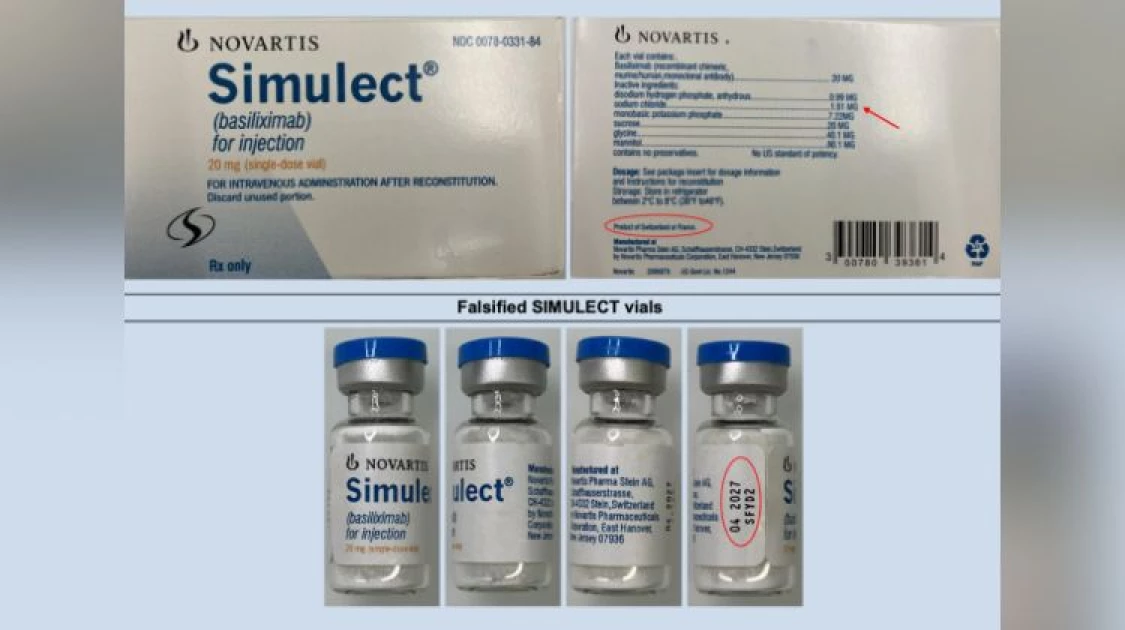
A photo of the original and falsified Simulect drug. Photo/PPB

Audio By Vocalize
Both drugs are essential for managing severe conditions, with SIMULECT used to prevent organ rejection in kidney transplant patients, and IBRANCE used for cancer treatment.
In a notice released by PPB Chief Executive Officer Dr. Ahmed Mohamed, the Board confirmed receiving alerts from the World Health Organization (WHO) concerning both counterfeit products, which have been reported in various international markets, including Rwanda, Bulgaria, and Türkiye.
The alert on IBRANCE, a cancer medication, concerns nine falsified batches. Four are confirmed counterfeit (FS5173, GS4328, LV1850, and TS2190) and five others are considered highly suspicious (GK2981, GR6491, GT5817, HJ8710, and HJ8715).
Physical inspection of the falsified IBRANCE revealed multiple deviations, including labels with spelling errors or poor-quality printing, security foil displaying the Pfizer logo in black ink, and capsules that are an unusual color, such as bright orange.
Laboratory analysis has since confirmed these falsified products contain no Active Pharmaceutical Ingredient (API), rendering them completely ineffective and unsafe for use.
The separate alert on SIMULECT, the critical immunosuppressant, displays the falsified batch number SFYD2.
Discrepancies on the counterfeit SIMULECT label include the use of the uppercase "MG" to list the ingredient dose, while the genuine product uses the lowercase "mg".
Additionally, the country of manufacture is falsely misrepresented as "Product of Switzerland or France," contradicting the genuine labeling of "Product of France."
The use of this falsified batch, which may contain incorrect, insufficient, or harmful ingredients, poses a serious risk to patient safety.
The PPB emphasized that the Board has not detected or confirmed the presence of any of the falsified IBRANCE or SIMULECT batches within the Kenyan market.
"To date, the Board has not detected or confirmed the presence of the falsified SIMULECT (basiliximab) batch SFYD2 within the Kenyan market," the statement read in part.
"This alert is therefore issued as a precautionary measure to enhance vigilance, prevent potential entry into the supply chain, and safeguard public health."
The Board urged all procurement agencies, hospitals, distributors, pharmacists, and the public to exercise extreme caution, remain vigilant, and immediately report any encounter with the falsified product batches.
Further, the Board emphasized the mandatory requirement that HPTs must be procured exclusively from licensed manufacturers, importers, distributors, and retailers.
PPB warned it would pursue legal action against any individual or entity involved in the distribution.

Leave a Comment